Protein Electrophoresis Electrophoresis is a simple yet effective tool that is used to separate proteins,…
Quantifying Proteins
Quantifying protein concentrations is necessary to understand the total protein content in a sample and is important for normalizing different protein samples for subsequent applications such as western blotting or immunoprecipitation. This is because assays such as these require precise total protein content results to provide accurate data.
There are several methods we can use to determine the protein concentration of our samples and the one you choose might depend on the downstream application that you have planned. In this post, we’re going to look at some of the most common protein measurement assays, their uses, and some of their limitations.
Direct absorbance measurement at 280 nm
One way of determining protein concentration directly is by measuring the absorbance of a sample at 280 nm. This is because aromatic amino acid residues such as Tryptophan and Tyrosine absorb UV light at 280 nm which enables us to calculate the protein concentration of a sample. One benefit of this method is that it doesn’t require any additional reagents so it is possible to reuse the extract after the quantification has been made. There are some limitations to this method though. It’s best for use with pure samples where the lass extinction coefficient is known or can be calculated from the amino acid sequence. Using mixed samples can give inaccurate results.
Colorimetric protein measurement: Bradford
Colorimetric assays are another option for determining protein levels. The Bradford protein quantification is one such colorimetric assay and it relies on the association of Coomassie Brilliant Blue R-250 with proteins and the shift in absorbance from 470 nm to 595 nm that this causes. In this assay, the sample is mixed with the Bradford reagent and left for 5 minutes before reading the absorbance at 595 nm. A standard curve with known protein amounts is used as a reference for the protein assay and is used to calculate the concentrations of your unknown samples. While bovine serum albumin (BSA) is most commonly used as a standard, it could be beneficial to use a reference protein that closely reflects your sample to improve the accuracy of your measurements.
The Bradford assay has lots of plus points. For example, it is less susceptible to interference by non-protein contaminants, such as salts. It also only includes one step so is quick to perform and doesn’t require any expensive machinery. On the downside, one of the biggest disadvantages of this method is that it doesn’t work if detergents or surfactants are in the sample.
Colorimetric protein measurement: BCA
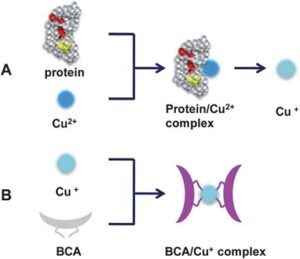
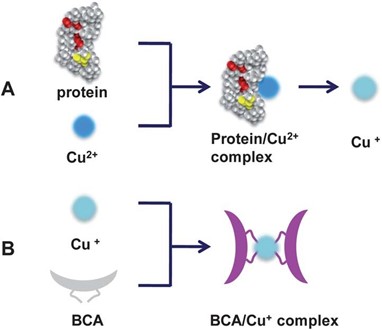
The bicinchoninic acid assay (BCA) is a copper-based assay that relies on the peptide bonds found in the proteins reducing Cu (II) to Cu+. Two molecules of BCA then chelate to each Cu+ ion which causes a change of color from green to purple with a strong absorbance at 562 nm. This assay also requires a standard curve for calculating protein quantification. The BCA assay is one of the most sensitive assays and can be used to detect proteins at concentrations as low as 5ug/ml. Unfortunately, it is susceptible to interference by some chemicals that can be present in protein samples which can make it less reliable.
The BCA assay relies on the peptide bonds found in the proteins reducing Cu (II) to Cu+. (Image via OneLab)
Colorimetric protein measurement: Lowry assay
This colorimetric assay also relies on the reduction capability of proteins. In the Lowry assay, Cu (II) sulfate is reduced to Cu+ and visualized using the Folin-Ciocalteu reagent. The use of this reagent results in a blue complex that can be quantified by measuring the absorbance between 500 and 800 nm. This technique also needs a standard curve that is measured in parallel to help determine the protein concentration of the samples. This assay is highly sensitive but many common substances such as carbohydrates and reducing agents interfere with it.
Fluorescent protein measurements
Fluorescent-based protein quantification methods are much more sensitive that the other methods we’ve described, which means that more protein sample is available for downstream applications. These assays are particularly useful when colorimetric assays cannot be used. Some of the advantages of fluorescent-based methods are that there are few steps involved in the process and the timing of these is not critical so the assays can be adapted for automated handling in high-throughput applications.
How Can We Help?
Cepham Life Sciences carry a broad selection of products that can be used in protein quantification. For more information have a look at our website or contact one of our team who will be happy to answer all your questions.
Contact Cepham Life Sciences Today!
Cepham Life Sciences team is available to meet all your proteomics and molecular biology laboratory needs. We have a wide range of quality research products, and our team of experts is on hand to offer advice and answer any questions that you have. You can reach us by phone at (410) 636-4954 or Toll-Free at 1-800-257-1565 (USA/CANADA) or by email.
Follow us on Twitter, Facebook, Google+, and LinkedIn to keep up to date on all of our products and promotions.