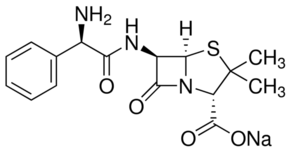
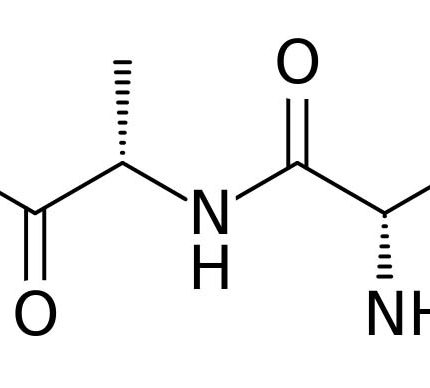
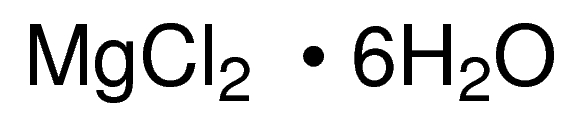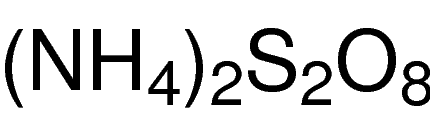General description
Magnesium chloride is a colorless crystalline solid also referred to as chloromagnestite, and is very hygroscopic in nature. It is soluble in both, water and alcohol and can be prepared by heating hydrated magnesium chloride crystals in a current of dry hydrogen chloride or by heating magnesium ammonium chloride. Anhydrous magnesium chloride crystallizes from magnesium chloride hexahydrate as leaflets. It is electrically conductive. The crystal structure of MgCl2 is layer like. The structure is cubic close packing with alternate layers of octahedral holes occupied by Mg2+ ions.
Synonyms: Magnesium chloride hexahydrate
CAS Number: 7791-18-6
Grade: Anhydrous
Molecular Formula: MgCl2 • 6H2O;
Molecular Weight: 203.30 g/mol
InChI Key: TWRXJAOTZQYOKJ-UHFFFAOYSA-L
Form: Powder
Boiling Point: 1412°C/1 atm (lit.)
Melting Point: 714°C (lit.)
Density: 2.32 g/mL at 25°C (lit.)