General description
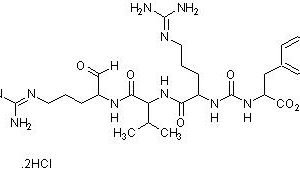
Chymotrypsin is a serine protease isolated from bovine pancreatic extracts. The selectivity of this enzyme is important for reproducible protein digestion and mass spectrometry (MS)-based protein identification. When compared to trypsin, chymotrypsin associated digestion typically generates a large number of shorter peptides. The endoproteinase chymotrypsin specifically cleaves at the carboxyl side of tyrosine, phenylalanine, tryptophan and leucine. Two very similar (80% homology) and predominant forms of chymotrypsin, A and B, are found in equal amounts in bovine pancreas, however, they have different proteolytic characteristics. Chymotrypsin can tolerate mild denaturing conditions, such as 0.1% SDS, 2M urea, 2M guanidine·HCl, 1% CHAPS, and 30% acetonitrile with optimal enzymatic activity.
Molecular Weight: 25 kDa
pH: 7.5 to 8.5
Form: Lyophilized powder
Storage temp.: -20°C, stable for 1 year




![SDS-PAGE Sample Loading Buffer [6X]](https://cephamls.com/wp-content/uploads/2019/02/SDS-PA1-2-scaled-430x430.jpg)




![IEF Cathode Buffer (pH 3-7) [10X]](https://cephamls.com/wp-content/uploads/2019/02/10498-3-430x334.jpg)