General description
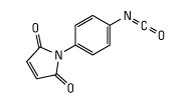
EMCS and its water-soluble analog Sulfo-EMCS are heterobifunctional cross-linkers that contain N-hydroxysuccinimide (NHS) ester and maleimide groups for covalent conjugation of amine- and sulfhydryl-containing molecules. NHS esters react with primary amines at pH 7-9 to form amide bonds, while maleimides react with sulfhydryl groups at pH 6.5-7.5 to form stable thioether bonds. In aqueous solutions, hydrolytic degradation of the NHS ester is a competing reaction where the change in rate is proportional to pH changes. The maleimide group is more stable than the NHS-ester group but will slowly hydrolyze and also lose its reaction specificity for sulfhydryls at pH values > 7.5. Therefore, conjugation experiments involving these cross-linkers are usually performed at pH 7.2-7.5, with the NHS-ester (amine-targeted) reaction being accomplished before or simultaneous with the maleimide (sulfhydryl-targeted) reaction.
Synonyms: EMCS, N-succinimidyl 6-maleimidocaproate, N-(ɛ-Maleimidocaproyloxy)succinimide, 6-Maleimidocaprioic acid N-succinimidyl ester
Name: N-(E-maleimidocaproyloxy)-succinimide
Molecular Weight: 308.29
Color & Form: White-beige solid
Melting Point: 70-73° C



![EMCS ([N-(E-maleimidocaproyloxy)-succinimide ester]) EMCS ([N-(E-maleimidocaproyloxy)-succinimide ester])](https://www.cephamls.com/wp-content/uploads/2019/02/EMCS.jpg)