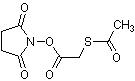
N-succinimidyl-S-acetylthioacetate (SATA) is a protein modification agent, used to protect free amine residues in proteins and peptides. SATA serves as a both a protecting agent for amine groups and as a useful reagent for converting an amine to a sulfhydryl. This can be very useful when looking to obtain a more specific handle for further protein modification or conjugation. Once N-succinimidyl-S-acetyl-thioacetate (SATA) is conjugated with the protein or other amine containing compound, the protected amine is easily converted to an unprotected thiol (-SH) residue by the reaction with hydroxylamine
SATA (N-succinimidyl S-acetylthioacetate) adds sulfhydryl groups to proteins and other amine-containing molecules in a protected form. The modified molecule can be stored indefinitely and treated with hydroxylamine to expose the labile sulfhydryl group when needed for conjugation reactions. SATA contains an N-hydroxysuccinimide (NHS) ester, which forms a stable, covalent amide bond with primary amines (i.e., lysine residues and the amino termini of proteins) and releases NHS as a by-product. De-protection (deacylation) to generate a free sulfhydryl is accomplished using hydroxylamine-HCl.
Sulfhydryl groups present on proteins, peptides and other compounds are important in protein chemistry/modification reactions. Frequently, thiols are unavailable or absent within the molecules of interest. Several reagents and techniques are available for introducing sulfhydryl groups or disulfides into proteins and peptides, including Traut’s Reagent, variants of SPDP, and variants of SATA.
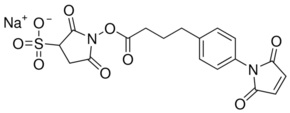
Molecular Formula: C8H9NO5S
Molecular Weight: 231.23
Spacer Arm: 2.8 Å
CAS Number: 76931-93-6
Synonyms: N-succinimidyl-S-acetyl-thioacetate; SATA Protein Modifier; SATA; Lysine Protecting Agent; N-Succinimidyl (acetylthio)acetate, N-Succinimidyl S-acetylthioglycolate, S-Acetylthioglycolic acid NHS ester; S-Acetylthioglycolic acid N-hydroxysuccinimide ester
Appearance: White to Off-White Solid
Storage: 4-8 °C, protected from moisture
Features of SATA(N-succinimidyl-S-acetylthioacetate):
1. Functional groups: NHS ester (amine-reactive) and sulfhydryl (protected)
2. Short-chain (2.8 Å) reagent – for covalent modification of primary amines and addition of a protected yet exposable sulfhydryl group, enabling heterobifunctional crosslinking strategies.
3. Adds a protected sulfhydryl that can be deprotected by hydroxylamine
4. Allows long-term storage of the sulfhydryl-modified molecule
5. Forms cleavable disulfide bonds with other sulfhydryl-containing molecules
6. Reacts with primary amines (e.g., lysine residues proteins) to form stable amide bonds
7. Preserves protein activity with its mild, non-denaturing reaction conditions




![IEF Anode Buffer [50X]](https://www.cephamls.com/wp-content/uploads/2019/02/10496-3-430x334.jpg)
![IEF Cathode Buffer (pH 3-7) [10X]](https://www.cephamls.com/wp-content/uploads/2019/02/10498-3-430x334.jpg)