General description
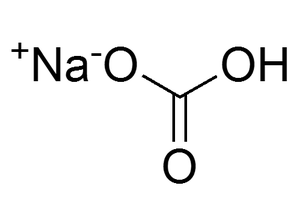
Sodium chloride (NaCl), commonly referred to as table salt, is an ionic water-soluble salt. It occurs naturally in soils and participates in diverse cellular functions within the human body. Sodium chloride has been routinely used for the preparation of tris buffered saline, phosphate buffered saline, MPM-2 (mitotic protein monoclonal 2) cell lysis buffer, immunoprecipitation wash buffer, LB (Luria-Bertani) media and dialysis buffer.
CAS No.: 7647-14-5
Molecular Formula: NaCl
MW: 58.44
InChI Key: FAPWRFPIFSIZLT-UHFFFAOYSA-M
pH: 5.0-9.0 (25°C, 5% in solution)
Melting Point: 801°C (lit.)