General description
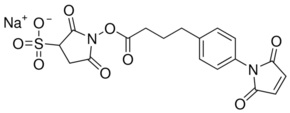
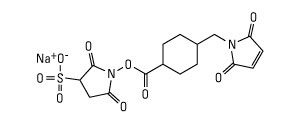
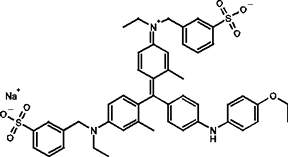
SMPB and its water-soluble analog Sulfo-SMPB are heterobifunctional crosslinkers that contain N-hydroxysuccinimide (NHS) ester and maleimide groups that allow covalent conjugation of amine- and sulfhydryl-containing molecules. NHS esters react with primary amines at pH 7-9 to form amide bonds, while maleimides react with sulfhydryl groups at pH 6.5-7.5 to form stable thioether bonds. In aqueous solutions, hydrolytic degradation of the NHS ester is a competing reaction whose rate increases with pH. The maleimide group is more stable than the NHS-ester group but will slowly hydrolyze and also lose its reaction specificity for sulfhydryls at pH values > 7.5. For these reasons, conjugation experiments involving these crosslinkers are usually performed at pH 7.2-7.5, with the NHS-ester (amine-targeted) reaction being accomplished before or simultaneous with the maleimide (sulfhydryl-targeted) reaction.
Features and Benefits:
- Reactive groups are Sulfo-NHS ester and maleimide
- Reactive towards amino and sulfhydryl groups
- Extended chain length limits steric hindrance
- Non-cleavable and water-soluble (compare to SMPB)
- Membrane impermeable, allowing for cell surface labeling